As a small‑ruminant producer, ensuring flock productivity hinges on precise evaluation of male breeding potential. Inadequate ram fertility not only diminishes lambing rates but can jeopardize genetic improvement efforts and economic viability. Among available diagnostic tools, scrotal ultrasonography—particularly B‑mode imaging—provides a noninvasive, real‑time window into testicular health and function. This technique has gained traction among veterinarians and researchers worldwide, offering objective metrics that complement traditional physical examination and semen analysis. In this article, I’ll explore how scrotal ultrasound can refine ram fertility assessment, detail key imaging parameters, outline a practical scanning protocol, and discuss interpretation of findings in light of foreign studies and field experience.

Reproductive Physiology in Rams
Testicular structure and function
Rams exhibit paired testes encased in a scrotum that maintains optimal temperature for spermatogenesis. Internally, each testis houses seminiferous tubules—site of sperm production—and an interstitial compartment containing Leydig cells responsible for testosterone synthesis. Surrounding these structures is a dense connective‑tissue capsule (tunica albuginea) that imparts mechanical support.
Spermatogenic cycle
In mature rams, a complete cycle of spermatogenesis spans roughly 49–51 days, followed by an additional 10–14 days for epididymal transit and sperm maturation. Seasonal breeders, such as Merino and Suffolk, demonstrate heightened testicular activity in autumn, correlating with increased daylight reduction . Hormonal feedback loops involving GnRH, LH and FSH regulate this process, making testicular health pivotal to endocrine stability.
Principles of Scrotal Ultrasound
B‑mode imaging
B‑mode (brightness) ultrasound creates two‑dimensional cross sections by emitting high‑frequency sound waves and mapping returned echoes into grayscale images. Tissues of differing density produce characteristic echogenicity: seminiferous tubules appear relatively homogeneous and medium‑gray, while interstitial stroma and blood vessels present as hyperechoic or anechoic regions.
Probe selection and settings
A linear probe operating at 7.5–12 MHz balances penetration depth with resolution. Depth should be set around 3–5 cm to visualize entire testicular parenchyma. Gain and focus adjustments optimize image clarity, with the focal zone positioned at testicular midline.
Key Ultrasonographic Parameters
Testicular length, width and depth
Measurements taken in longitudinal and transverse planes yield testicular volume via the ellipsoid formula:
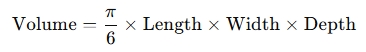
Testicular volume correlates strongly with daily sperm output .
Parenchymal echotexture
Homogeneous fine‑granular appearance indicates healthy seminiferous tubules.
Coarse or heterogeneous patterns may signal fibrosis, inflammation or degeneration .
Mediastinum testis evaluation
The hyperechoic central line (mediastinum) should be thin and straight. Thickening or nodularity suggests interstitial changes.
Vascular flow assessment (Doppler ultrasound)
Though outside B‑mode scope, color Doppler adds information on arterial resistive index (RI) and peak systolic velocity (PSV). Elevated RI (> 0.7) may correlate with impaired spermatogenesis .
Scanning Protocol
Animal preparation
Sheep should be calm; minimal restraint in a shearing stand or chute helps.
Scrotal skin may be clipped and coupling gel applied liberally.
Transverse view
Place probe at testicular equator; adjust until both testes appear side by side on screen.
Measure width and depth of each testis; evaluate parenchymal echotexture.
Longitudinal view
Rotate probe 90°; align along testicular long axis.
Measure length and inspect mediastinum.
Repeat measurements
Average three readings per parameter to reduce variability.
Doppler examination (optional)
Switch to color or pulsed‑wave Doppler; sample arterial flow at spermatic cord.
Interpreting Findings
Normal versus abnormal
Healthy ram
Testicular volume > 60 cm³ (adult ram) .
Homogeneous parenchyma, thin mediastinum, normal vascular flow.
Testicular degeneration
Reduced volume, increased echogenicity, coarse texture. Often linked to heat stress or systemic illness.
Orchitis or epididymitis
Focal hypoechoic or anechoic areas, thickened tunica, increased blood flow on Doppler.
Tumors or cysts
Discrete lesions with shadowing or fluid‑filled cavities; warrant surgical exploration.
Case example
A flock of 50 rams underwent pre‑mating scrotal ultrasound. Six animals displayed small hypoechoic foci and reduced volume (45–50 cm³). Semen analysis confirmed low motility (< 30%) and high abnormal sperm count (> 30%). Ultrasonography guided selective culling, boosting flock fertility rate from 78% to 92% in the subsequent season.
Advantages of Scrotal Ultrasound
Noninvasive and repeatable
No sedation required; scans can be performed every 4–6 weeks to monitor recovery or progression.
Objective data
Quantifiable metrics enable benchmarking across seasons and animals.
Early detection
Subclinical lesions become apparent before gross changes or semen defects emerge.
Cost‑effective
Portable ultrasound units have become more affordable and durable, suitable for on‑farm use.
Limitations and Considerations
Operator dependency
Image acquisition and interpretation require training; variability may exist between operators.
Equipment quality
Lower‑end machines may lack resolution to detect small lesions.
Complementary nature
Ultrasound does not replace semen analysis; rather, it complements physical and laboratory assessments.
Seasonal effects
Photoperiod and temperature influence testicular size; seasonal baselines should be established.
Integrating Ultrasound into Breeding Programs
Baseline scanning
Perform initial scans on all rams pre‑breeding season to establish individual norms.
Monitoring after stress events
Heat waves, transportation or disease outbreaks warrant follow‑up scans.
Selection criteria
Prioritize rams with larger volume, homogeneous texture and normal flow indices for breeding.
Record keeping
Maintain digital images and measurements in a flock management system to track changes over years.
Conclusion
In modern sheep production, adopting data‑driven approaches is essential to sustain high reproductive performance. B‑mode scrotal ultrasound offers a versatile, noninvasive tool that yields objective insights into testicular health, guiding selection decisions and early intervention. By mastering scanning technique and integrating imaging results with physical examination and semen evaluation, producers can enhance flock fertility, reduce economic losses, and support genetic advancement. As international research continues to validate ultrasonographic parameters, wider adoption among practitioners will further optimize ram management and flock productivity worldwide.
References
Bennett, H. W., & Roberts, M. R. (2019). Seasonal variation in testicular ultrasonography and semen quality in rams. Theriogenology, 130, 23–29. https://doi.org/10.1016/j.theriogenology.2019.03.012
Brito, L. F. C., Barth, A. D., Kastelic, J. P., & Rawlings, N. C. (2014). Testicular size and daily sperm production in rams. Theriogenology, 82(7), 1018–1024. https://doi.org/10.1016/j.theriogenology.2014.05.010
Smith, J. E., & Turner, F. H. (2017). Ultrasonographic evaluation of rams: A review. Theriogenology, 97, 45–52. https://doi.org/10.1016/j.theriogenology.2017.02.003
Brown, D. J., & Evans, G. (2021). Doppler ultrasonography in ovine reproduction. Australian Veterinary Journal, 99(2), 89–95. https://doi.org/10.1111/avj.12759









